Kugako Sugimoto, NOST Tokyo
Origineel gepubliceerd op de site van Agentschap NL.
Japan wil snel voortgang maken met medische en farmaceutische toepassingen van stamcellen. De overheid heeft nu de eerste klinische trial van de transplantatie van een retina, die opgebouwd is met behulp van geïnduceerde pluripotente stamcellen, goed gekeurd. Ook is er toestemming gegeven voor experimenten waarin onderzoekers menselijke organen in dieren laten groeien. De overheid wil bovendien een Japanse versie van de Amerikaanse National Institutes of Health opzetten, die zich vooral gaat richten op deze stamcellen. Ruimer gebruik van stamcellen in immunotherapieën en het screenen van kandidaat-geneesmiddelen wordt eveneens een belangrijke onderwerp van onderzoek. In deze nieuwe ontwikkelingen participeren overheid, bedrijfsleven en academische instellingen.
Applying iPS cells in Japan
Summery
Japan is acting quickly to realize medical and pharmaceutical applications of iPS cells. The Japanese governments approved the first clinical trial of the transplantation of retina made of iPS cells and experiments to grow human organs in animals.In addition, a Japanese version of the National Institutes of Health to promote medical and pharmaceutical research will be established, which focuses on iPS cells. Wider use of iPS cells such as immunotherapy and drug screening is also investigated. The government, academia, and business are involved in this movement.
Detail
Since Professor Yamanaka of Kyoto University co-won the Nobel Prize in Physiology or Medicine in 2012 for his development of iPS cells, the Japanese government has acted quickly to realize the use of iPS cells for regenerative medicine and drug creations. One of the plans is to build a Japanese version of the National Institutes of Health (NIH) of the United States.1 The institute controls budgets and leads the medical and clinical research according to the national policy. The Japanese prime minister and his cabinet proposed to build a Japanese version of NIH, partially hoping that iPS research and its application create a boost for the Japanese economy. The domestic market of regenerative medicine would be about twenty nine billion euro in 2050, according to the Ministry of Economy Trade and Industry. Clinical trials and other research related to iPS cells have rapidly received green light to go ahead. Presently various research related to iPS cells is being actively conducted in medical, biological, pharmaceutical, logistics, and robotics industries and academia in Japan. However, there is a concern that basic sciences that do not deliver quick financial returns, like medical and pharmaceutical application of iPS cells, might be left behind.
Approval of growing of human organs in an animal
While growing human organs for transplantation is expected to realize regenerative medicine, it is always associated with the ethical issues of growing human cells in animals Until now, Japan has not approved implanting an fertilized animal egg that has human cells back into the animal’s uterus. However, due to the development of technology to control the growth of animals and to produce specific organs, an ethical committee of Council for Science and Technology Policy approved the research of the creation of human organs in an animal.2 Making animal organs in a different animal has already been successful. A mouse with the pancreas of a rat has been created. The same technology can be used to create human organs in an animal soon. The government will work on the revision of the current research policy by incorporating the suggestions of the committee soon. This new policy may enhance the creation of human organs by using iPS cells in an animal body.
However, the committee suggested imposing certain regulations to grow sperm, egg, and cranial nerves in an animal and to use primates because of the highly ethical issues involved. In addition, each research project needs an individual approval from the government.
First clinical trial of iPS cells
The government committee approved the world’s first clinical research of iPS cells for transplantation of retina on July 12, 2013. On July 19, 2013, the Minister of Health Labor and Welfare approved this clinical trial very quickly. Transplantation would be conducted in the summer of 2014.3
A research team of Dr. Masayo Takahashi of RIKEN in Kobe will conduct the small-scale, open label* pilot study of the safety and feasibility of transplantation of autologous human iPS cell-derived retinal pigment epithelium cell sheets. First, cells will be obtained from the patients with neovascular age-related macular degeneration**. Then, they are grown into retinal pigment epithelial cells. Cells will be tested for their safety to make sure there is no risk for iPS cells to grow into a tumor and for their quality. Then, they are transplanted into the affected retina. Since the main purpose of this research is to check the safety for clinical application, patients with advanced symptoms of age-related macular degeneration with less risk of aggravation will be chosen. A big improvement of eyesight cannot be expected by this pilot study. The research team will conduct the intensive long-term safety monitoring after the transplantation. The operation would be conducted at Kobe City Medical Center General Hospital.
iPS cells for larger beneficiaries
The primary use of iPS cells is to restore functional organs and tissues. However, the number of patients is small compared to the larger population of the patients with other diseases. Therefor a wider use of iPS cells is also being developed.
Immunological application
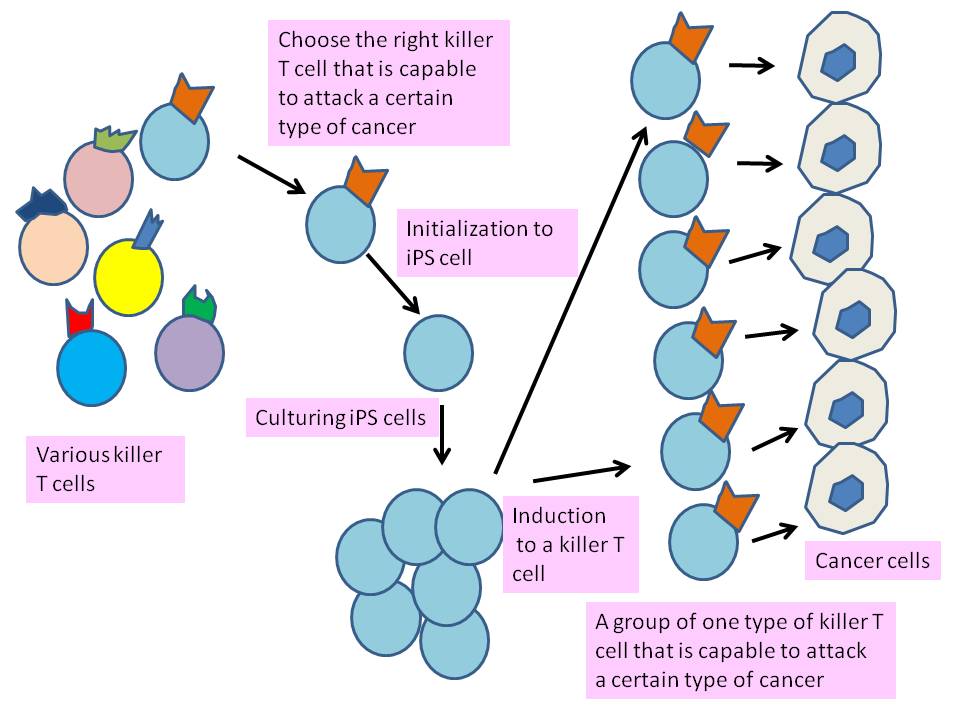
Professor Hiroshi Kawamoto of the Department of Immunology and Cell Biology, Graduate School of Biostudies of Kyoto University and his team are working on the creation of killer T cells*** to attack cancers by creating iPS cells from specific types of T cells.4
The point is that the team used a particular and fully developed T cell that can attack a particular type of cancer. During the developing into a killer T cell in human body, in a thymus, cutting and pasting of genes (gene rearrangement) to express T cell receptors occurs. T cell receptors recognize cancers. Consequently, each fully developed killer T cell has different genes. If iPS cells are made from the right and fully developed Killer T cell and they are induced into killer T cells again, such induced killer T cells can all attack a particular (targeted) type of cancer (Fig. 1). This is because they express receptors to recognize a particular cancer.
In other words, if iPS cell is made from a somatic cell and is induced into killer T cells. Only one portion of such killer T cells attack a particular type of cancer.
Fig. 1 Creation of killer T cells from iPS cell, chosen from various type of killer cells. (Idea obtained from JST announcement, Jan 5, 2013)
In current immunotherapy, cancer cells often overwhelm killer T cells. Working effectively to attack cancer, abundant and specific killer T cells are needed during the treatment. Large and steady supply of killer T cells that attack a targeted cancer by using iPS cells would enhance the results of immunotherapy.
In the study, the team used the T cell JFK6 that responds to the antigen of human malignant melanoma. This T cell expressed CD3 (as a T cell marker) and CD8 (as a Killer T cell marker). Then, the team made it into iPS cells by introducing Yamanaka factors**** (Oct3/4, Klf4, Sox23, c-Myc) to JFK6. The created iPS cell has an exact set of genes to recognize a human malignant melanoma. Then, this iPS cells are co-cultured with feeder cells and developed into premature T cell that express CD8 and CD4 (as a helper T cell***** marker). Further, the team added antibodies to react with CD3 to stimulate the development of T cell receptors. Finally, T cells expressed CD8 (as a killer T cells marker) and the team was able to obtain a large amount of killer T cells that attack human malignant melanoma.
Professor Kawamoto further aims to get iPS cells fully induced in the thymus in a patient’s body, where premature T cells are further differentiated and gain specificity in nature. By this strategy, the process of culturing in vitro and transplantation of differentiated iPS cells would be omitted.
For Regrowth of hair
Dr. Manabu Ohyama of Keio University School of Medicine succeeded in growing hair on mice by co-transplanting precursors of keratinocytes created from human iPS cells and fibroblasts of young mice.5 Signals of human cells were found in the formed hair follicles in the transplanted area. Advanced baldness is currently treated by the transplantation of skin with abundant hair, such as lower back of a scull to the targeted area such as a forehead. However, such transplantation is limited due to the shortage of source. Transplantation of another way is to produce transplantable cells by co-culturing of keratinocytes and dermal papilla cell. However, during the co-culturing, keratinocytes and dermal papilla cells tends to lose the ability to create hair follicle. iPS cells, on the other hand, have pluripotency and excellent proliferation ability. Dr. Ohyama mentions the importance of using precursors of keratinocytes was a key to successful growth of hair instead of using fully differentiated keratinocytes.
For Drug Discovery
According to The Nikkei of July 23, 2013, Chugai Pharmaceutical Co., Ltd is going to use iPS cells to screen drugs that might cause side effects.6 This method will decrease costs of the development of new drugs. Chugai plans to create cardiac muscles cells from iPS cells. Side effect such as irregular pulses caused by the candidate drugs will be tested. Chugai plans to use iPS cells for screening in 2016.
Japanese Version of NIH
The government agreed at the meeting of all the cabinet members on August 9, 2013 that a new Japanese version NIH would receive a budget for scientific research of about seven hundred seventy million euro from the budget currently scattered over various ministries.1 Since the budget for life science is about twenty three hundred million euro (2013), one third of the budget goes into a new Japanese version of NIH.
NIH has three major roles. One is to control scientific funding. The second is to lead the scientific themes reflecting national policy. The third is to transfer the research results for industrial use, including the provision of data of clinical trials and research results through an accessible database.
High expectations of the clinical and pharmaceutical application of iPS cells seem to facilitate the building of a Japanese version of NIH although the main targeted diseases of NIH are infectious disease, cancers, psycho-neurologic disease, intractable disease, and rare diseases. The Japanese version of NIH would provide favorable environments for iPS cells research which goes well with the government’s aim to boost the the use of iPS cells by medical and pharmaceutical industries. Japan is a huge importer of medical equipment and would like to reverse this situation to become a successful exporter of medical devices, drugs, and medical equipment including iPS cells applications.
However, academics have concerns that top down control of medical and pharmaceutical research would focus on limited number of research projects that produce profit quickly. Such an approach by controlling budgets and policies by the government might underestimate the importance of basic research and its diversities. Academics think a diversity of basic research will lead a few but excellent discoveries and inventions. As a matter of fact, iPS cells were produced in such a study environment. A group of presidents of major Japanese universities suggested that multiple representatives from academic medical/clinical researchers should join the promotion headquarters of NIH to reflect the academic approach for transfer of new technology to industries.
Summary
Ever since Professor Yamanaka of Kyoto University co-received the Nobel Prize for his creation of iPS cells, medical and pharmaceutical industries have become restless and full of expectations. This synchronized the economic stimulation strategy of the current Japanese government. Studies on iPS cells to realize clinical use are making progress with the support of rapid governmental approvals. However, academias have concerns that continuous development cannot be achieved if the government only focuses on the quick economic returns. The balance of applied studies and basic studies of science is important for Japan, today and in the future.
Ever since Professor Yamanaka of Kyoto University co-received the Nobel Prize for his creation of iPS cells, medical and pharmaceutical industries have become restless and full of expectations
Sources
- Nikkan Kogyo Shinbun (Aug 9, 2013)
- Mainichi Shinbun (Aug 4, 2013) (in Japanese)
- RIKEN Research
- JST announcement (Jan 4, 2013) (in Japanese)
- Keio University Press Release (in Japanese)
- The Nikkei of July 23, 2013 (in Japanese)
**neovascular age-related macular degeneration (WIKIPEDIA)
***killer T cell (cytotoxic T cell) (WIKIPEDIA)
****Yamanaka factor (WIKIPEDIA, reprogramming)
*****helper T cell (WIKIPEDIA)