Kugako Sugimoto, NOST Tokyo
originally published on the site of NL Agency
Summary
To prepare for the ageing society and the creation of a prominent new industry, Japan is trying to produce new biomedical materials leading to revolutionary medical treatments. The Japan Science and Technology Agency (JST) is offering grants, called S-innovation, to support the development of revolutionary medical treatments for the next decade. It emphasizes the commercialization of research output. There are already applications of effective biomedical materials on the Japanese market.
Details
Introduction
Japan is developing new biomedical materials for revolutionary medical treatments ten years from now. To support this development, the Japan Science and Technology Agency (JST) provides up to 70,000,000 yens per year for ten years for individual research teams as an S-innovation Grant. There are three reasons why the Japanese are looking for new biomedical materials. Firstly, Japanese society is rapidly ageing, secondly they want to foster a prominent industry for its export and tax paying potential. Thirdly and finally, there is demand for easy-to-use medical devices that usually require biomedical materials.
Ageing Society
Japan is rapidly ageing. The ratio of people who are older than 64 in the total population was 22.9% in 2009 and is expected to become 33.7% in 2035. This development increases the gross national medical expenditure. To reduce the medical expenditure requires cost effective medical treatments more than ever.
Prominent Medical Industry
The medical industry, including medical services, may be expected to be a good future tax source for the Japanese government. Expectations for future tax income from previously pivotal industries in Japan such as cars and electric products are not too high due to current the worldwide economic slowdown and the appearance of newcomers on the world market. While the government tries to contain domestic medical expenditures, the growing domestic market and export of biomedical materials, medical devices and medical services might open new financial sources for Japan. Since ageing society is not only an issue for Japan but also for other developed countries, the market is worldwide. The Japanese government also started to promote international medical tourism to Japan. High quality and revolutionary medical treatments will be an asset for the medical tourist sector.
Demand for easy-to-use devices
Easy-to-use medical devices are wanted by doctors. Today most doctors are very busy due to a shortage of medical doctors. In addition, learning to use new devices makes them constantly busier. Furthermore, home care takers will also need easy-to-use medical devices more often, because the Japanese government recently shifted the strategy for the care for the elderly from hospital-centered to home-based.* Home care takers and maybe elderly people themselves need to use medical devices.
S-innovation encourages the research leading to such products as biocompatible catheters and guide wire, light and durable implants, super engineering plastics, extra-corporeal circulation, and regenerative medicine. Some examples will make clear what medical devices using biomedical materials were recently developed in Japan.
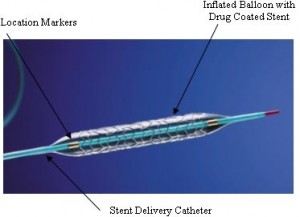
Stent
A stent is a mesh-like tube that supports the blood vessels from the inside to keep them open after interventional treatment with a catheter and a balloon (Fig. 1). Sometimes a stent causes restenosis; cells accumulate inside the implanted stent as a result of inflammation due to an immune reaction. There are mainly two solutions for restenosis. One is application of drugs to the stent to decrease the accumulation of cells. The other is the removal by autonomous disappearance of a stent after its necessary period of use.
Fig. 1 A stent (Wikipedia)
Drug-eluting stents (DES)
Professor Kensuke Egashira of Faculty of Medical Sciences of Kyushu University developed a stent that releases nucleic medicine to prevent restenosis. This is called a drug-eluting stent (DES). He uses the 7ND gene, which restrains the expression of MCP, a major factor of inflammation. Nano particles of this are mixed with polyvinyl alcohol. The particles are coated with biodegradable polylactic acid (PLA) in order to prevent too quick a release of the content. The coating also controls the charge of the particle surface, which makes endocytosis of the particles by targeted cells easier. Egashira’s research team observed the expression of 7ND genes and no in-stent restenosis.
NF-κB Decoy (which restrains the transcription factor, NF-B, responsible for inflammation) was also tested as a candidate for DES. The results seemed good.
Bioabsorbable stents
In general, there are two kinds of bioabsorbale materials. The first one is made of the polymer poly-L-lactic acid (PLLA) and the other is made of metal. The PLLA stent by the Kyoto Medical Planning Co., Ltd., called an IGAKI-TAMAI stent, will be degraded into H2O and CO2 and be absorbed after two to three years after implantation. Advantages of polymer stents over metal stents are the applicability to patients with metal allergies and the easy mixing of drugs into the stents.
The company Sentan Iryo Kaihatsu produced a stent made of a Mg-Ca alloy, made from necessary metals for the human body. It will gradually disappear in the body due to corrosion. The retention time in the body can be controlled by the ratio of Ca in the alloy. Metal stents are excellent in strength, plasticity, and recoil prevention. Prof. Egashira works with Sentan Iryo Kaihatsu to create DES that will completely disappear from the vessels after a certain critical period.
DDS
A Drug-Delivery System (DDS) aims at delivering drugs to the locations where the drugs should work at the right time. Enveloping materials for drugs are key to optimize the required function of a DDS. The envelope should prevent drugs from being absorbed or being degraded before the drugs reach the targeted area. In addition, the gradual collapse of encapsulation is required in order to release of drugs for a prolonged period.
A DDS of nucleic acid drugs has to be stable and has to contain the drugs inside the capsule when they are still outside the targeted cells. When nano devices enter the targeted cells, they form endosomes surrounded by endosome membrane in the cytoplasm of the targeted cells. Then the nano devices need to break the endosome membranes and to get out of the endosome in order to reach the nucleus of the cell where a nucleic acid drug works.
The teams of Prof. Kazunori Kataoka of the Department of Materials Engineering at the University of Tokyo and Prof. Hideyoshi Harashima of the Hokkaido University met these requirements of a DDS by using the chemical reactions dependent on pH. The research teams produced a DDS by using high molecular micelle of self-organization of polymers and a Multifunctional Envelope-type Nano Device (MEND) respectively.
Micelles
The micelles Prof. Kataoka’s team created were made of a combination of homo-polymers of poly{N-[N’-(2-aminoethyl)-2-aminoethyl]aspartamide} (PAsp(DET)) and block-polymers of PAsp(DET) and polyethylene glycol (PEG) (Fig.2). PAsp(DET) changes the structure according to the acidity of its surroundings as well as its ability to attack the membrane. PAsp(DET) does not attack the cell membranes outside the cells in neutral condition. However, once they enter targeted cells and are surrounded by the membrane of endosomes, they are able to break the membrane of the endosome thanks to the low pH inside the endosome. Then the drugs in the micelle can be released into the cytoplasm.
Fig. 2 How to get out of endosome of nano-device made of PAsp(DET-Aco) charge inversion polymer (Final Research Report)
In the core of the micelle, PAsp(DET) and plasmid DNA (pDNA) forms polyplex, a complex of cationic polymers and DNA. SH-group inserted to the side chain of PAsp(DET) makes disulfide bonds inside the polyplex and offers stability on the route trip to the targeted cells. When the device enters the cytoplasm of the targeted cells, disulfide bonds of the polyplex are loosened and release pDNA because of a high concentration of glutathione in the cytoplasm.
Hydrophobic PEG located on the outer layer of the micelle also helps to make the trip to the targeted cells safer thanks to their stealth nature. PEG on the outer layer of the micelles, decreased the formation of destructive aggregates in blood. On the other hand, nano particles without PEG-coatings made aggregates due to the interaction of platelets. With intra-vital real-time confocal laser scanning microscopy using a modified Nikon A1R, the real-time behaviour of micelles and aggregates in blood could be detected.
The research results were good; they showed high gene expression. The researchers aim to apply the use of this nano micelle particle for medication for circulatory and vascular systems, respiratory systems, anti-cancer and musculoskeletal systems.
MEND
Another nano device using a micelle structure has outside layers of fusogenic membranes (Fig. 3). Nuclear drugs have to go through several membranes, including the nuclear membrane, to reach a nucleus. MENDs with fusogenic membranes are able to send nucleic acid drugs without using nucleic pores. The outer membrane is made of endosome membrane fusogenic lipid and inner membranes are made from nucleic membrane fusogenic lipid. Because of these two different types of layers, MENDs are able to go through different types of membranes and deliver the drugs into the nucleus.
Fig. 3 MEND (Final Research Report)
The research results of this type of MEND were positive. The expression of genes increased five hundred times in non-dividing dendritic cells in comparison with MEND without multiple layers of membrane around the nano device. Shionogi & Co., Ltd. and the team of Prof. Harashima are working on manufacturing of the device for commercial use.
Artificial bones
Nakashima Medical Co., Ltd. and the Professors Nakano and Yoshikawa of Osaka University developed an implant that causes bones to grow without making gaps between implants and bones. The implant has multiple ditches which direct apatite of bones and collagens to form mesh-like structures along the ditches for a good fit. Experiments with dogs were carried out with good result. The team is planning for market introduction in the near future.
Conclusion
It is difficult to define what ‘revolutionary’ medication is. However, a mindset aiming at ‘revolutionary’ must urge researchers to go the extra mile. One of the important goals of S-innovation is valorization of innovation, which means the research results should enter the market. Japan is considered weak in commercialization of research results. To overcome this, research team are encouraged to employ multiple specialists people such as researchers, technicians, medical doctors, production engineers and retailers. Japanese people really are awaiting their revolutionary medication to secure a stable life in a society that will age super fast in the near future.
Sources:
1) White paper on ageing society (2011) by Cabinet Office
2) Research Theme of S-innovation
5) Molecular mechanism of in-stent restenosis and novel treatment strategy of gene-eluting stent developed with bioabsorb-able nano-particle electrodeposition (2007). Folia Pharmacol. Jpn. 129:171-176
6) *NOST Tokyo News; Medische thuiszorgtechnologie en de overheid