Kugako Sugimoto, NOST Tokyo
Origineel gepubliceerd op de site van Agentschap NL
Samenvatting
Japan behoort tot een kleine groep van landen die in staat is nieuwe geneesmiddelen te ontwikkelen. Japan heeft een groot aandeel in de internationale – en de thuismarkt. In vergelijking met bedrijven en sectoren uit andere landen (zoals de VS en in Europa) zijn de R&D-kosten van Japanse farmaceutische bedrijven hoog. Het ontwikkelingstraject van nieuwe geneesmiddelen in Japan is vergelijkbaar met dat elders in de wereld. Echter, er is veel meer tijd nodig om het traject af te ronden. Door gebrek aan onderzoekers en vrijwilligers die nieuwe medicijnen willen testen komen belangrijke nieuwe geneesmiddelen langzamer op de markt. Om het proces te versnellen wil Japan de samenwerking op het gebied van klinisch onderzoek internationaliseren en het aantal onderzoekers opvoeren. Een andere oplossing wordt gezocht in een betere communicatie tussen farmaceutische bedrijven en de autoriteiten die geneesmiddelen toelaten en registreren.
Japanese Drug Creation Procedure Steps
Summary
Japan is one of a handful countries that is cable of producing new drugs. Japan has large international and domestic markets. R&D costs relative to the sales of the Japanese pharmaceutical companies are high compared to those of other major domestic industries and the competitors in US and EU. The Japanese production process of new drugs is similar to other countries. However it takes long for the process to finish due to shortages of investigators and patient volunteers, which makes serious drug introduction lagging. To speed up the process, international collaboration on clinical trials and an increasing number of investigators are solutions as well as frequent communication between pharmaceutical companies and exam authorities.
International
In 2011, the pharmaceutical market in the world was roughly seven hundred billion (700,000,000,000) euro and Japan accounted for eighty two billion (82,000,000,000) euro. Japan accounted for 11.7% of international pharmaceutical market, while the share was 36.2% and 4.7% for North America and Germany respectively. In 2011, four drugs originated from the Japanese companies ranked within the top 30 drugs in international market sales. Companies of Japan accounted for 12.3% of top 30 pharmaceutical companies in terms of sales and those of US and Switzerland accounted for 41.8% and 14.8% (respectively in 2011). Takeda, the largest pharmaceutical company in Japan, was ranked 12, Astellas ranked 17, Diichi Sankyo 19, and Otsuka holdings ranked 20.
Domestic
Domestic production was forty-eight billion (48,000,000,000) euro in 2010 and roughly 90% of the production came from ethical drugs. In 2009, the percentage of shipment of ethical drugs was 22.3%. 15.9% and 84.1% of the approved pharmaceutical drugs with new active ingredients in Japan came from Japan and overseas (respectively between 2008 and 2011). The amount of new drugs sold by Japanese companies between April 2008 and May 2012 has a total of 89 items. Half of them originated from Japanese companies.
The cost of R&D against the sales of the pharmaceutical companies was high compared to other industries in Japan. In 2010, the ratio of R&D cost was 12.02% for the pharmaceutical industry, while that of information and communication electronics equipment was 5.81% with the average of 3.93%. The average R&D ratio of top 8 companies in Japan was around 35-36% from 2008 to 2011, while it was 29% for US and EU. Japanese top 8 companies were Takeda Pharmaceutical, Otsuka Pharmaceutical, Astellas, Diichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Dainippon Sumitomo Pharma, and Shionogi. To be cost effective, mergers between Japanese pharmaceutical companies have been conducted. Examples are Astellas (merger between Yamanouchi Pharmaceutical and Fujisawa Pharmaceutical) in 2006 and Daiichi Sankyo (merger between Sankyo and Diichi Seiyaku) in 2008. Also, mergers with oversea pharmaceutical companies have been pursued.
Drug Creation Process
Japan is one of handful countries (such as US, Switzerland, and UK) which is capable of producing original drugs. The procedure of creation of original drugs in Japan is similar to those of other countries. It starts with the basic research followed by nonclinical study. Then, clinical trials that have three phases are conducted. If the results are good, a pharmaceutical company applies for approval and scrutiny by the Ministry of Health, Labour and Welfare. Once the scrutiny has been passed, the company will get the approval and moves to the stage of production and sales. After selling the new drug, a new investigation phase called Phase IV starts. This procedure from basic research to the production usually takes from 9 to 17 years with a huge amount of expenditure. It is said that only one in 30,000 candidate substances will become a product.
Basic research
Substances that have potential to become drugs are searched during basic research. Methods to obtain such substances are extraction from plants, animals, and microorganisms. Chemical synthesis with or without biotechnology is also used. Genome information is a recently available tool in this step. Nature and chemical structures of the candidates are studied and the candidates are narrowed down through multiple screening tests.
Nonclinical test
Efficacy and safety of the candidate substances that passed the basic research is tested in nonclinical test. Animals and cultures will be used in this test. Behaviors such as absorbance, distribution, metabolism, and discharge of the candidate substances are tested. Quality and stability of the newly created candidate substances are also tested.
Clinical Test
In this clinical test step, efficacy and safety of the candidate substances that pass the nonclinical test are tested by administering to humans. A clinical test step consists three phases. Phase I is a test against the health of a small number of people. Phase II is a test against a small number of patients. Phase III is a test against a large number of patients. Phase I tests side effects and safety. Phase II aims to find effective and safe administration methods as well as dosage. Phase III compares the efficacy with the pre-existing similar drugs on the candidates. The average period of clinical trials is from 3 to 7 years. Hospitals usually conduct clinical tests and data is analyzed regarding potentials as new drugs.
Application and Approval
With a positive result of clinical test, a company applies to get an approval as a product from the Ministry of Health, Labour and Welfare. Then, the Ministry asks Pharmaceuticals and Medical Device Agency (PMDA) for scrutiny of the drug. If the drug passes the test, the Pharmaceutical Affairs Food Sanitation Council gives an approval for production and sales. This application and approval period requires from 1 to 2 years. The prices of the drugs that are covered by the medical insurance would be determined according to the Drug Pricing System by the Ministry of Health, Labour and Welfare.
Survey in the market (Phase IV)
New drugs on the market will receive feedback from the consumers. Sometimes, side effects that did not appear during the development may emerge. Medical information personnel (MR) of pharmaceutical companies correct such information. This survey is conducted between 4 and 10 years after the drug was launched on the market. Other systems and the latest science may reevaluate and check the efficacy and safety of the new drugs.
Development of drugs takes a long time and lots of labour and money. Especially, development of drugs in Japan seems to take a long time. Some of the reasons for this long development are slow clinical trials, scrutiny and approval steps.
Slow clinical trials
Good clinical practice (GCP) enacted in 1998 was reinforced to adjust to the needs to meet the international standards of the clinical trials to facilitate the creation of drugs in Japan. GCP is an additional support for Pharmaceutical Affairs Act. In Japan, the shortage of highly skilled staff capable of coordinating the entire clinical trial process is a problem. The second problem is the shortage of patient participants in clinical trials. It is said that benefits to the participants under the universal health insurance coverage is not clear, while other countries provide free medical treatments for the participants who might not have enough medical insurances. In addition, contracts between hospitals and pharmaceutical companies especially on the expenditure of clinical trials are not clear.
Clinical trials need good planning and designing. A Contract Research Organization (CRO) supports to design and takes care of the progress of clinical trials. CRO handles the purposes, periods, necessary number of data, selection of patients, administration including dosage, measurement method, medical cautions and prohibition, provision of measures for side effects, and clinical statistics. In Japan, there are CROs such as Mitsubishi Chemical Medicine, CMIC, EPS, Paraxel, and SRL Medisearch.
Drug lag and measures
Drug lag is a delay before a new drug is allowed on the Japanese drug market, while the identical drug already appeared in other markets. To reduce the drug lag between Japan and overseas, pharmaceutical companies expect the number of judges of PMDA will be increased. The currently piled up applications would be materials to train new judges on-the-job. In addition, to speed up the clinical tests, pharmaceutical companies are encouraged to consult and discuss with PMDA more often during the designing of the clinical tests. Pharmaceutical companies should get information on what kind of data is needed for approval to prevent the surprise of additional tests at the last moment. Also, results obtained at each step of clinical trial can be discussed with PMDA before going to the next step. International cooperation on clinical trials is one of solutions to speed up clinical trials. This also aims to get approvals from both overseas and Japan simultaneously. Currently conducting clinical trials in Japan can be viewed at the web site of Japan Pharmaceutical Manufacturers Association though key word searches (http://www.clinicaltrials.jp/user/cteSearch_e.jsp).
Drug Pricing System
The prices of drugs are determined by Ministry of Health, Labour and Welfare in Japan. This applies drugs that are covered by the medical insurance system. The prices are determined by comparison with similar pre-existing drugs. If the new drug is not comparable to any other existing drugs, the price will be determined by the cost of production. Every two years, prices are reconsidered and tend to be lower. From 2010, some drugs in the periods of patent terms keep the same prices until the generic version of those drugs are sold.
Important examples of drugs originated in Japan
Prograf (tacrolimus)
Prograf (tacrolimus1) is an immunosuppressant drug and isolated from Actinomycete, (Streptomyces tukubaensis), found in the soil on the foot of Mt. Tsukuba near Tokyo. This is a byproduct of Actinomycete and 23-membered macrolide lactone2. This drug, developed by Astellas, is used for treatment of immune disease and also prevents rejection of transplanted organs. This drug suppresses lymphatic cells, but its restraining effect on bone marrows is not strong, resulting in small side effects.
Levofloxacin
Levofloxacin3 is a quinolone4 synthetic antibacterial drug, used for infectious disease by both gram positive and gram negative bacteria, Chlamydia, Mycoplasma, and Legionnaire. This drug prevents the duplication of DNA of microorganisms by affecting DNA gyrase5 and topoisomerase6 IV. The drug was developed by Daiichi Sankyo and now is sold as a generic medicine.
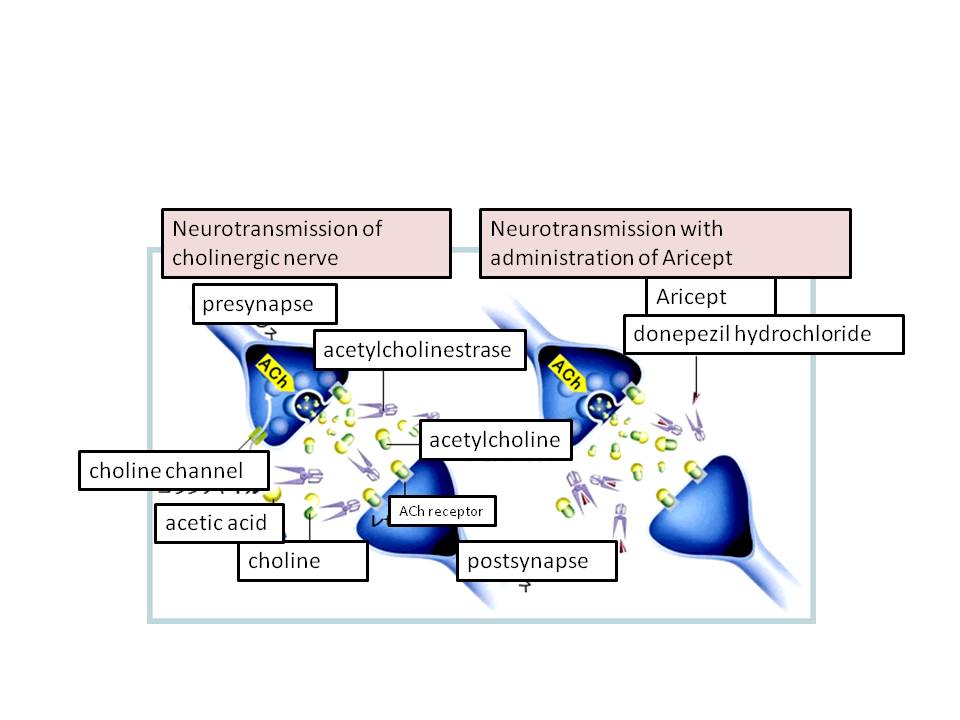
Aricept (donepezil hydrochloride)
Aricept (donepezil hydrochloride7) is a treatment medicine for minor cases of Alzheimer’s disease, developed by Eisai. The amount of Acetylcholine of patients with Alzheimer’s disease is low. Acetylcholine is a substance, which is very much involved in human memory. Acetylcholine interacts with the enzyme called acetylcholinesterase which degrades and decreases the amount of acetylcholine. The result is a low concentration of Acetylcholine in the brain (Fig 1). Donepezil hydrochloride (Aricept) blocks the reaction site of Acetylcholinesterase in which acetylcholine docks. This inhibition keeps a high concentration of Acetylcholine in the brain, which will help the neuron transmission of Cholinergic8 nerve and mitigates the symptoms of Alzheimer’s disease.
Fig. 1 Aricept intervenes acetylcholinestrase and prevents the decrease of acetylcholine in neurotransmission. (Aricept of Eisai)
Statin
Statin9 is a drug which decreases blood cholesterol. High cholesterol suggests increase of the risk of cerebral infarction and myocardial infarction. Akira Endo of Sankyo found mevastatin from blue mold in 1973. The effect on animal studies by using rats did not show good results failing in decreasing of blood cholesterol levels. However, the later study showed mevastatin worked well on chickens and dogs. Unfortunately, side effect of mevastatin was concerned and the development was stopped. However, pravastatin, one of related products of mevastatin, finally came on the market in 1989.
Conclusion
Japan is one of countries that is able to produce new drugs and it has large international and domestic markets. Recent challenges to create new competitive drugs are slow process of approval of new drugs in Japan. Solutions are adopted, such as promotion of communication between pharmaceutical companies and PMDA and Ministry of Health, Labour and Welfare, to reduce unnecessary obstacles. It is also pointed out that the number of investigators of exam authorities should increase. International collaboration for clinical trials and international mergers seem to be tools for Japanese companies to survive by decreasing the high cost of R&D.
Sources
- Japan Pharmaceutical Manufacturers Association (JPMA)
- Japan CRO Association (JCROA)
- Presentation material on Pharmaceutical Industry Vision 2013 by Ministry of Health, Labour and Welfare (in Japanese)
- Aricept of Eisai (in Japanese)
Words:
1 tacrolimus (Wikipedia)
2 macrolide lactone (Wikipedia)
3 Levofloxacin (Wikipedia)
4 Quinolone (Wikipedia)
5 DNA gyrase (Wikipedia)
6 Topoisomerase (Wikipedia)
7 Aricept (Donepezil) (Wikipedia)
8 Cholinergic neuron (Wikipedia)
9 Statin (Wikipedia)