Kugako Sugimoto, NOST Tokyo
Origineel gepubliceerd op de site van Agentschap NL.
Regeneratieve geneeskunde is sterk in opkomst. Onderzoeksactiviteiten op het terrein van stamcellen en weefsel nemen sterk toe. Tijdens opslag en transport van cellen en weefsel moet de functionaliteit en kwaliteit gewaarborgd zijn. Aangepaste en nieuwe logistieke technologieën moeten de oplossing daarvoor bieden.
In dit artikel komen de volgende onderwerpen aan de orde
- Uitvindingen van nieuwe containers voor transport, nieuwe bevriezingstechnieken en nieuwe opslag- en bewaartechnieken van medische en biologische materialen.
- Slimme logistiek is belangrijk voor zeer geavanceerde medische behandelingen.
Research and Development of Logistics for Cells and Tissues in Japan
Summary
Demands for regenerative medicines, stem cell research, and iPS cells development involve technologies for storage and delivery of cells and tissues. Success as a new industry to use and sell stem cells and tissues depends on the technology to maintain viability of cells and tissues during transport and delivery. New inventions and modified technologies for new applications to keep cells/tissues fresh are emerging in Japan.
Introduction
Logistics for medical and biological research and industries require a lot of careful material handling because materials are alive and fragile. The viability of the delivered materials is a key to medical and biological logistics. Temperature, physical shock, positional change, pH, structural destruction by ice crystallization, chemicals, and pressure damage the quality of biological materials. Especially for regenerative medicine, keeping viability of cells and tissues during the storage and transportation determines the success of transplants to patients. In Japan, regenerative medicine offered at the clinic uses the patients’ own cells. The first step is to send these cells to a cell processing center (SPC). After processing and culturing of the ready-made cells, cells need to travel back to the patients for transplant. Success as an industry for regenerative medicine including iPS cells that Professor Yamanaka of Kyoto University developed, involves new logistics in addition to the medical and biological research. Special containers, freezing methods, and antifreeze solutions to maintain the viability of cells, tissues, and samples are emerging in Japan.
Containers
Transport of biological samples is usually carried out at a low temperature in a medium. However, for regenerative medicine, cultures and cells processed and incubated at a SPC may require the same SPC culturing conditions during transportation in order to keep cells and tissues viable and functional.
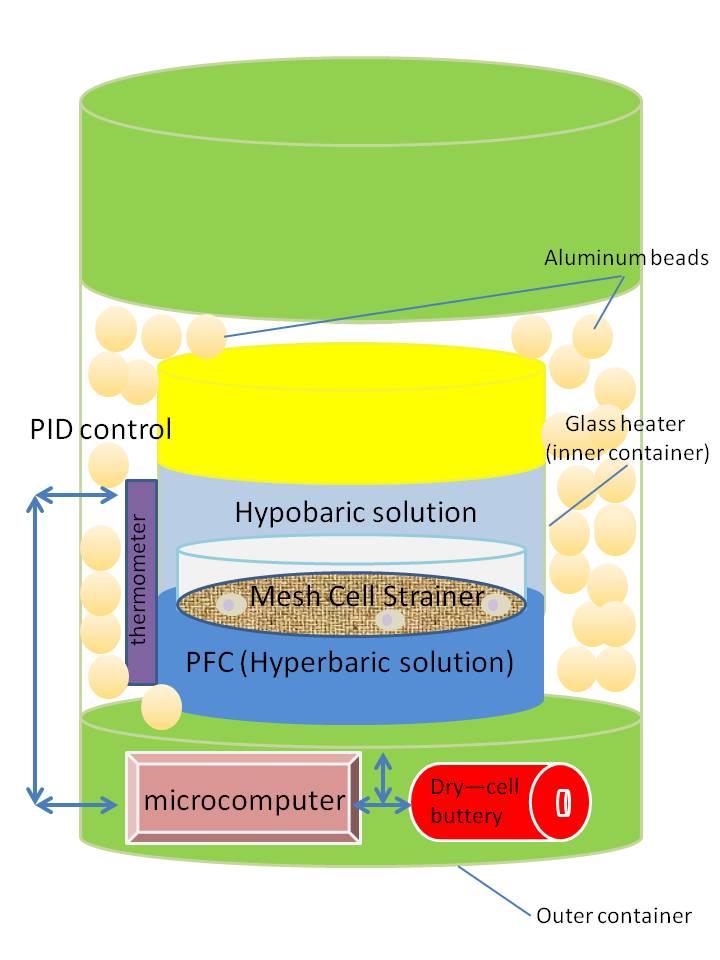
Dr. Norihiko Hata of Laboratory of Medical System Engineering of Tokyo Denki University developed a container that provides temperature usually used for culturing cells. The container has a cell strainer made of a nylon mesh that enhances the recovery rate of cultured cells and absorbs physical shocks from the outside of the container. The container is made of two layers (Fig. 1). The inner container is a glass heater. The outer container is made of metal. The bottom part of the inner container contains high-density perfluorocarbon (PFC) and the upper part is filled with a hypobaric solution. A cell strainer holds cells at the density boundary of these two liquids. The density difference of these liquids stabilizes the cells by preventing their moving around in the inner container. PFC also helps to recreate the incubation conditions such as temperature and pH during the transportation by controlling gas in the liquids. Proportional-integral-differential (PID) controlling is used to stabilize the temperature in the inner glass heater. Aluminum beads fill the space between inner glass heater and the outer metal containers as insulators. Storage experiments with the container with cells for 72 hours at 25 ̊C and 37 ̊C have shown good survival rates of around 90%, while the survival rate at 8 ̊C was less than 70%. In addition, a strainer increased the harvest rate (>80 %) compared to a conventional centrifugation (<40%).
Fig. 1 Image of the container that consists of inner and outer containers. (Idea obtained from the paper by Dr. Hata et al.)
Hitachi Transport Systems Ltd. also developed a container that kept the temperature at 37 ̊C for 45 h and was suitable for long distant transportation. It tested the effect of transportation of cultured cells by train for 5 h at 37 ̊C. Transplant of these cultured cells to animals was successfully done after storage during this period in the container.
A research team of Kyoto University developed a container that maintains a sterile condition and horizontal position of samples by combining a metal container that Hitachi Transport Systems and partners previously created (Fig. 2). The inner container is autoclavable. Setting samples into the inner container can be handled in a sterile environment such as on a clean bench. A mechanism like a gyroscope keeps samples in the inner container horizontally. The outer container keeps an optimal temperature for the samples and gives physical protection. In addition, it protects against a change of atmospheric pressure in case of transportation by air. Delivery of ready-made cells by an airplane will be commonly used to shorten travelling time from the CPS to the patients.
Fig. 2 Pictures of the container. Inner container is autoclavable. Outer container provides physical protection. (Kyoto University)
Freeze
Freezing technology used for frozen food will be applied for medicine including regenerative medicine. Frozen food industries have tried to minimize the loss of freshness and taste in frozen food and they usually focused on minimizing the formation of ice inside food. Ice crystals in food destroy the cell structure, especially cell membranes. When cells of food are defrosted, the nutrients and tasty chemicals leave the cells and drip through the destroyed cell membrane. Ice formation inside the food occurs in a certain temperature range. To avoid a large amount of ice formation, rapid freezing to minimize the exposure time to this temperature range is one way. The other way is to go through the critical temperature range without letting ice be formed. Once water goes out from the critical-ice-formation-temperature range to the lower temperature, water makes smaller size of ice in the combination with a rapid freezing. The result is frozen food with all its tastes intact.
Cells Alive System (CAS)
ABI Inc., invented technology called “Cells Alive System (CAS)” that suppresses the volume of ice formation during the crystallization inside food. The technology seems to use magnetic field to make water molecules oscillate during freezing food, which creates the condition of super-cool. Then rapid freezing is applied to food to freeze completely. However, the details of technology are not disclosed.
The company is collaborating with various research teams on the application of CAS technology to medical treatments such as adult-living-donor liver transplantation, tooth bank to store periodontal membranes for autograft, and stem cell storage. In the experiment using live mice, legs of mice frozen using CAS technology for 10 minutes regenerated into functional tissue, blood vessels, nervous system, and bones after defrosting the frozen legs. However, legs frozen with dry-ice or liquid nitrogen did not recover. Damage by freezing looks smaller by CAS compared to conventional freezing methods.
Microwave-Vacuum Dehydration
Kyushu Institute of Technology is developing technology to reduce the volume of ice formation during freezing by pre-removal of water from food. This technology is called microwave vacuum drying method. Freezing is initiated at a lower temperature in dehydrated food compared to the untreated food. Untreated tuna started to freeze at around 0 ̊C and the super-cooling of the dehydrated sample started to freeze at -6 ̊C.
The freezing initiation point decreased according to the smaller water content in food because the solute concentration in food increased. In addition, pretreatment removal of water made possible to form smaller ice crystal and lose less dropping-loss of taste and nutrients. This technology could be developed for future methods of storing cells and tissues for medical and pharmaceutical use.
Antifreezing solution
One method to avoid the damage of freezing is the addition of antifreeze solution. Conventionally, dimethyl sulfoxide (DMSO) and glycerin are used. These solutions decrease water loss through cell membrane and help to keep cell structures intact. However, DMSO is highly toxic to cells. When cells are used for treatments or experiments, DMSO should be removed completely. There are other safe additives such as antifreeze proteins and antifreeze glycoproteins. However, these are very expensive. Safe, affordable, and effective antifreezing solution are needed.
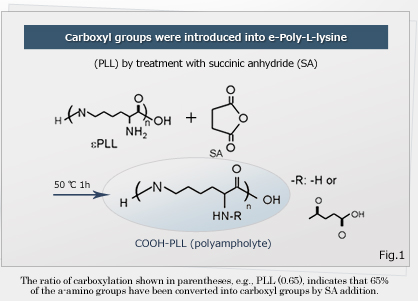
Associate Professor Kazuaki Matsumura of Japan Advanced Institute of Science and Technology and a research team of University Kyoto developed an inexpensive and safe antifreeze solution. They changed some amino groups in poly (L-lysine) to carboxyl group to create polyampholyte*, with the final ratio of amino groups to carboxyl groups of 50:50 ~ 80:20 (mol:mol)(Fig.3). In an experiment, L929 cells obtained from mice are suspended in different concentrations of a poly (L-lysine) derivative solution for 6 h at -80 ̊C with cell concentration of 1 x 106 cells /ml. Then, the viability was examined by staining cells with Trypan Blue after thawing in a water bath at 37 ̊C. Survival efficiency was 95% in a solution of 50mol% of amino groups carboxylated in polyampholyte. It is suggested that ampholyte inhibit ice recrystallization by causing a non-colligative freeze point depression** at low concentrations by binding to ice crystals. Human mesenchymal stem cells also showed good survival rates without addition of serum during freezing. The researchers suggest that storage and freezing with polyampholyte should be applicable for iPS cells due to low toxic effects.
Fig. 3 Structure of created polyampholyte. (Japan Advanced Institute of Science and Technology)
Pressure
Applying high pressure (50 – 1,000 MPa) to store food and microbes without freezing has been known. Professor Akio Shimizu of Soka University has tried to develop a device that preserves cells by applying weak pressure during the transportation (Fig. 4). Weak pressure less than 10 MPa seemed to stabilize the cells without freezing. Temperature during the transportation was set higher than the crystallization temperature of water in the cell and less than the optimum temperature of the cells. Incubation experiment of glial cells obtained from a newborn rat showed relatively good survival after one-day storing at 4 ̊C with 0 – 10 MPa. The same experiment at 37 ̊C with 10 MPa was also O.K. This storage method needs to be developed further by clearing negative effects of pressure and length of storage to cells as well as finding the right combination of length of incubation, temperature, and strength of pressure. In addition, the container needs to be smaller for a practical use to carry cells.
Fig. 4 Description of system to give pressure to the cells in an inner container. (Soka University)
Conclusion
Medicine and biological research have always involved freezing, storage, thawing, and transportation of cells and samples for measurements of their activities. Highly advanced medical treatment such as regenerative medicine requires technology to keep top-shape viability of cells and tissues, which might be lost during the steps of transportations. Containers that keep the optimal position, temperature for cells and tissues, freezing technology including antifreeze solution are being invented. Affordability and easy handling of the invention will be also important in addition to the technology for viability of cells/tissues. In addition, combination of individual inventions with other logistic systems such as tracking and temperature controlled storage at the airport will be important to succeed as medical/pharmaceutical/biological industry.
[Streamer]
However, for regenerative medicine, cultures and cells processed and incubated at a SPC may require the same SPC culturing conditions during transportation in order to keep cell cells and tissues viable and functional.
References:
- Yoshimi Memorial T.M.P Grant report
- Hitachi Transport System, Ltd.
- Research Information of Kyoto University
- Cell Alive System
- Improvement of freezing quality of food by pre-dehydration with microwave-vacuum drying
- Polyampholytes as low toxic efficient cryoprotective agents with antifeeze protein properties
- Patent (Soka University)
**non-colligative properties (Wikipedia)
**freezing point depression (Wikipedia)